Subissue of Cell: Discovery of Enterobacteria Degrading Uric Acid
The original Singularity Cake Singularity Network is included in the collection # 62 Intestinal Microbes # 57 Cardiovascular Diseases.
* For medical professionals only.


It’s summer. Have you arranged all the beer skewers and juice in little seafood? Singularity cake every time I watch this kind of food electronic mustard tuber, the barrage will float past the "gout package!" Hmm … Everyone has a high awareness of health!
Foods such as beer, seafood and animal offal are rich in purines, and some purines, such as xanthine, hypoxanthine and uric acid, the final metabolites of purines, are considered as uremic toxins (wastes that patients with impaired renal function cannot metabolize normally) [1], which are related to some symptoms of chronic kidney disease [2].
It is also a typical symptom of gout that uric acid can not be metabolized normally, resulting in the formation of pro-inflammatory crystals deposited on joints. However, some studies have found that uric acid concentration within the solubility range can also promote atherosclerosis through AMPK pathway-mediated inflammation [3]. Isn’t the kidney working hard enough? However, intestinal microbes will shoot!
Recently, the research team at the University of Wisconsin-Madison found that intestinal microorganisms can affect the progression of atherosclerosis by regulating the level of uric acid circulation. They identified intestinal microorganisms that use purines such as uric acid as carbon and energy sources in anaerobic environment, as well as gene clusters encoding key steps of purine degradation. This is a potential new way for intestinal microorganisms to affect health, which may provide a new way for treating gout or preventing cardiovascular diseases. The research results were published in Cell, Host and Microorganism [4].

Previous studies have shown that the atherosclerosis burden of more than 100 inbred mice in the Hybrid Mouse Diversity Group (HMDP) is very different, and the composition of intestinal flora is also obviously different [5,6].
In this study, the researchers selected four kinds of HMDP, two of which showed obvious atherosclerosis (experimental group) and two of which showed almost no signs of disease (control group), transplanted their flora to aseptic APOE knockout mice (which could spontaneously form hypercholesterolemia and atherosclerosis) and fed them with a diet supplemented with 0.2% cholesterol for 8 weeks.
Compared with the control group, the pathological changes in the experimental group are obvious. Traditional cardiovascular risk factors such as body weight and cholesterol intake, as well as intestinal microbial metabolites lipopolysaccharide, trimethylamine -N- oxide (TMAO) and short-chain fatty acids can’t explain this difference.
Macrogenome analysis identified 1649 functional features and 52 bacterial taxonomic features, and correlation analysis identified several bacterial functions related to atherosclerosis load, including those related to purine production and transformation. Among the 682 metabolites in mouse plasma, purine metabolites, including xanthine, xanthine nucleoside, inosine and uric acid, are positively correlated with the size of atherosclerotic plaque.
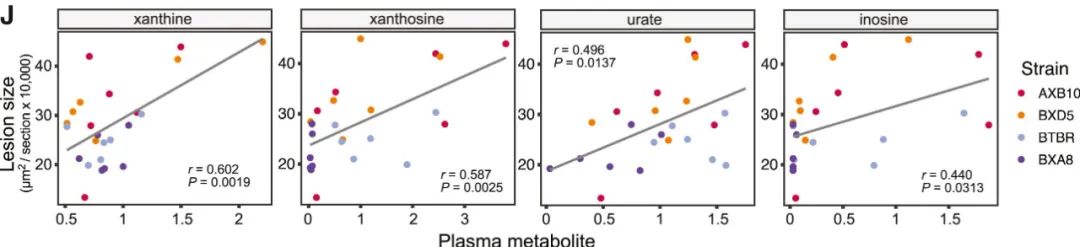
Correlation between xanthine, xanthine nucleoside, uric acid and inosine and atherosclerotic plaque size
In the cohort of human volunteers, there is a significant positive correlation between coronary artery calcification (CAC) score and uric acid level, and several taxonomic units in Clostridium have a negative correlation with uric acid level, which may affect uric acid level.
Compared with conventional mice, most purine levels in cecum/feces of sterile mice decreased, and only uric acid and allantoin levels increased. Similarly, the level of uric acid in plasma of sterile mice also increased significantly. Therefore, intestinal microorganisms may be able to regulate the level of purine in intestine and whole body. This strengthened the researchers’ determination to isolate purine-degrading bacteria in the intestine.
Intestine is the key organ of purine homeostasis. Dietary purine is absorbed in the intestine, and intestinal microorganisms produce and recover purine needed for its anabolism. About 30% uric acid produced by body metabolism will be secreted into the intestine.
The researchers tried to enrich anaerobic bacteria on the culture medium with uric acid as the main source of carbon and energy by using human feces samples, and obtained several bacteria from Firmicutes and Proteobacteria, which were identified as Enterocloster bolteae and Escherichia coli by 16S rRNA sequencing. The model strain ATCC BAA-613 of Enterocloster bolteae was cultured in a medium supplemented with 12mg uric acid. 10ml of strain culture degraded 49.6μmol of substrate within 24 hours, and produced 106.9μmol of acetate.
Based on the success of this small-scale attempt, the researchers expanded the search for intestinal microorganisms with purine degradation ability, and identified 34 kinds of bacteria from 6 phyla by the same method. The purine utilization ability of different bacteria is different. For example, Escherichia coli MS200-1 and I-11 have stronger uric acid utilization ability than Escherichia coli K12. Allantoin can support the growth of Enterocloster bolteae and Escherichia coli, but it cannot support the growth of Clostridium difficile or Edwardsiella tarda.
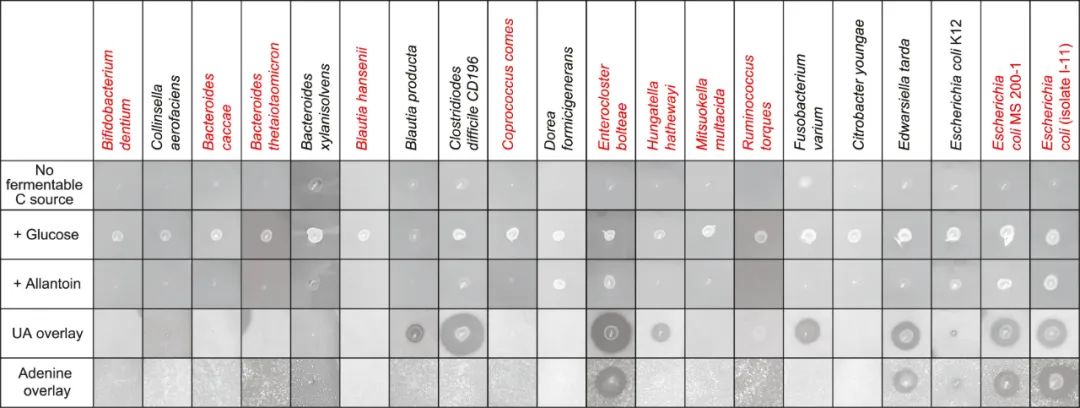
Representatives of 34 kinds of bacteria
By comparing the conserved chromosome regions of these intestinal microorganisms, the researchers found that five genes (dpaL, hydA, ssnA, ygeY and xdhD) were conserved. Compared with the sterile mice with non-purine degrading flora and the sterile mice with conserved gene deletion mutant flora, the plasma uric acid level of the mice with wild-type strains was significantly lower. There was a negative correlation between the level of purine-related metabolites in cecum and the abundance of five genes in APOE knockout sterile mice. This shows that these genes and their encoded intestinal bacteria have the ability to regulate uric acid homeostasis in vivo.
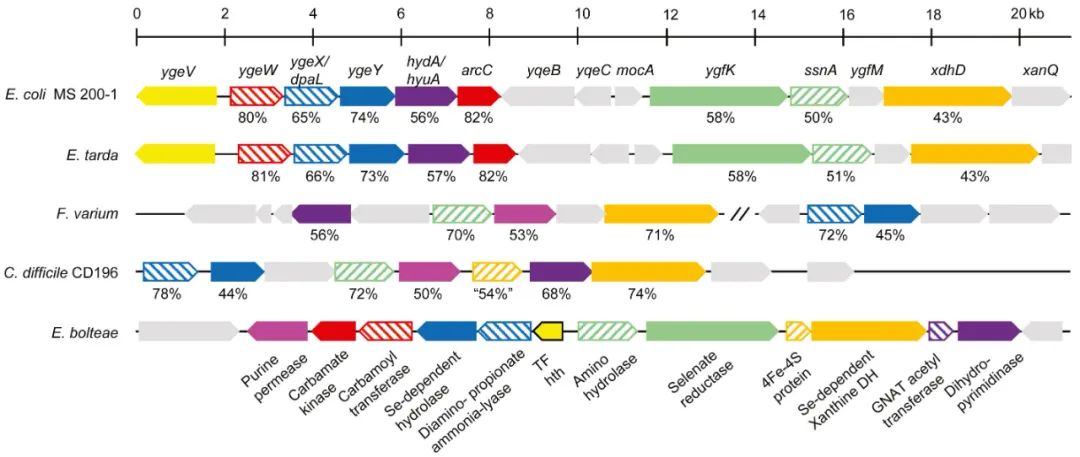
Identification of conserved genes
Generally speaking, this study found a previously neglected factor affecting uric acid circulation level-intestinal microorganisms and the corresponding key genes. In the future, more research is needed to answer the effects of aerobic and anaerobic purine consumption pathways on purine and intestinal microecology, as well as atherosclerosis and other health conditions.
# Friends, in order not to miss the important research progress of Singularity Cake Push, be sure to add stars to us ~
References:
[1] Falconi C A, Junho C V C, Foga?a-Ruiz F, et al. Uremic toxins: an alarming danger concerning the cardiovascular system[J]. Frontiers in physiology, 2021, 12: 686249.
[2] Valkenburg S, Glorieux G, Vanholder R. Uremic toxins and cardiovascular system[J]. Cardiology Clinics, 2021, 39(3): 307-318.
[3] Kimura Y, Yanagida T, Onda A, et al. Soluble uric acid promotes atherosclerosis via AMPK (AMP-activated protein kinase)-mediated inflammation[J]. Arteriosclerosis, thrombosis, and vascular biology, 2020, 40(3): 570-582.
[4] Kasahara K, Kerby R L, Zhang Q, et al. Gut bacterial metabolism contributes to host global purine homeostasis[J]. Cell Host & Microbe, 2023.
[5] Bennett B J, Davis R C, Civelek M, et al. Genetic architecture of atherosclerosis in mice: a systems genetics analysis of common inbred strains[J]. PLoS genetics, 2015, 11(12): e1005711.
[6] Org E, Parks B W, Joo J W J, et al. Genetic and environmental control of host-gut microbiota interactions[J]. Genome research, 2015, 25(10): 1558-1569.


The author of this article should be Yu Yan.
Read the original text